Synthesis of α‐Trifluoromethylated Ketones from Vinyl Triflates in the Absence of External Trifluoromethyl Sources - Kawamoto - 2017 - Angewandte Chemie International Edition - Wiley Online Library

Vinyl triflates derived from 1,3-dicarbonyl compounds and analogs: access and applications to organic synthesis - ScienceDirect

Radical Desulfur-Fragmentation and Reconstruction of Enol Triflates: Facile Access to α-Trifluoromethyl Ketones. | Semantic Scholar

Vinyl triflates derived from 1,3-dicarbonyl compounds and analogs: access and applications to organic synthesis - ScienceDirect

PDF) Zinc triflate-mediated cyclopropanation of oxindoles with vinyl diphenyl sulfonium triflate: a mild reaction with broad functional group compatibility
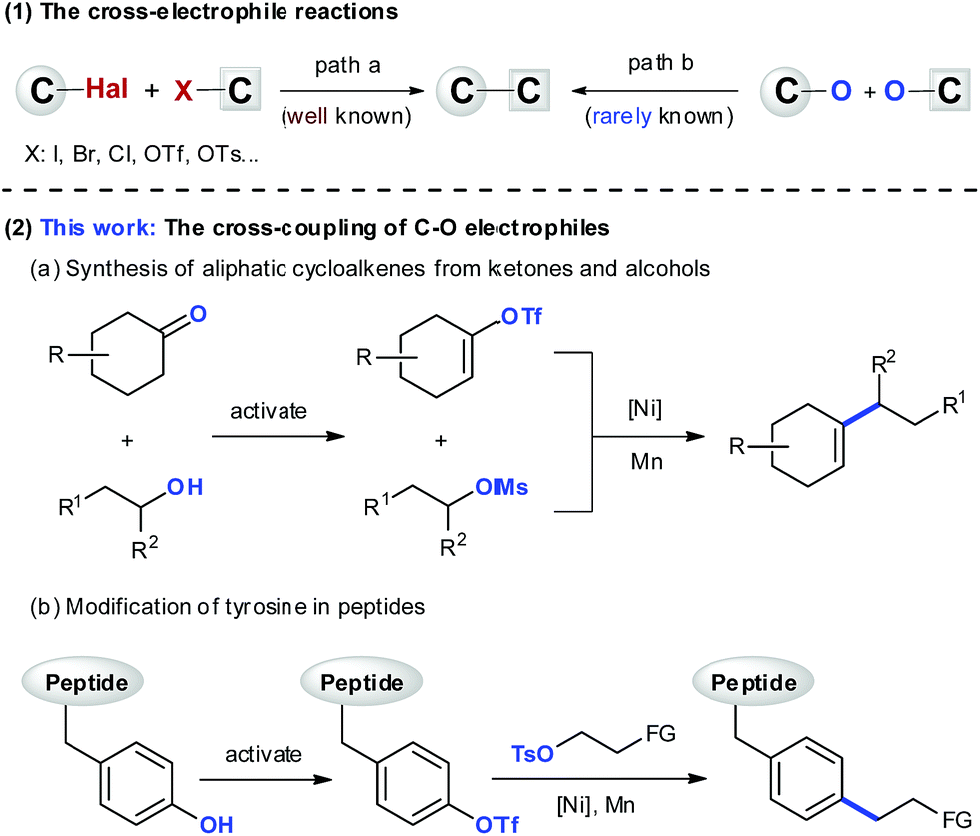
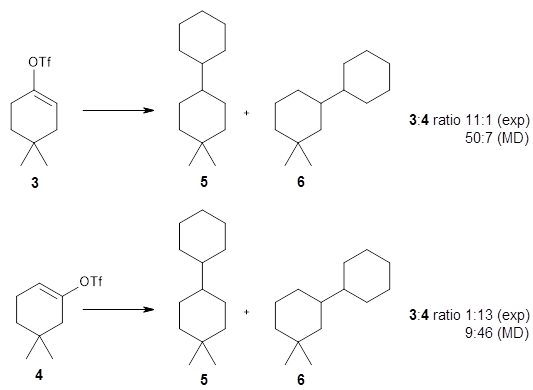
Ni-catalyzed cross-electrophile coupling between vinyl/aryl and alkyl sulfonates: synthesis of cycloalkenes and modification of peptides - Chemical Science (RSC Publishing) DOI:10.1039/C9SC03347E
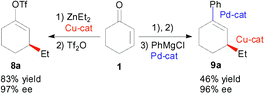
Tandem one pot asymmetric conjugate addition–vinyl triflate formation–cross coupling methodology - Organic & Biomolecular Chemistry (RSC Publishing)

Vinyl triflates derived from 1,3-dicarbonyl compounds and analogs: access and applications to organic synthesis - ScienceDirect

Vinyl triflates derived from 1,3-dicarbonyl compounds and analogs: access and applications to organic synthesis - ScienceDirect
Pd-catalyzed synthesis of α,β-unsaturated ketones by carbonylation of vinyl triflates and nonaflates - Chemical Communications (RSC Publishing) DOI:10.1039/C9CC02210D

Palladium-catalyzed conversion of aryl and vinyl triflates to bromides and chlorides. - Abstract - Europe PMC

Vinyl Triflate–Aldehyde Reductive Coupling–Redox Isomerization Mediated by Formate: Rhodium‐Catalyzed Ketone Synthesis in the Absence of Stoichiometric Metals - Shuler - 2019 - Chemistry – A European Journal - Wiley Online Library

Nickel-Catalyzed Cross-Electrophile Vinyl–Vinyl Coupling: An Approach to Structurally Versatile Dienylboronates | CCS Chem

Vinyl Triflate–Aldehyde Reductive Coupling–Redox Isomerization Mediated by Formate: Rhodium‐Catalyzed Ketone Synthesis in the Absence of Stoichiometric Metals - Shuler - 2019 - Chemistry – A European Journal - Wiley Online Library

Palladium-catalysed coupling of vinyl triflates with enynes and its application to the synthesis of 1α,25-dihydroxyvitamin D3 - ScienceDirect